Digital CAPA process management for COVID playbook

Capacity Building via CAPA process is much more than training and includes:
Institutional and legal framework development, making legal and regulatory changes to enable organizations, institutions and agencies at all levels and in all sectors to enhance their capacities. Increase in overall efficiency of the department along with better service delivery, attitudinal change of employees – towards safety culture improvement. Integration of CAPA in the safety culture implements Environment Health & Safety (EHS) rules to an extent, procedures and laws which companies require to track all recordable events relating to workplace illness, injury or death.
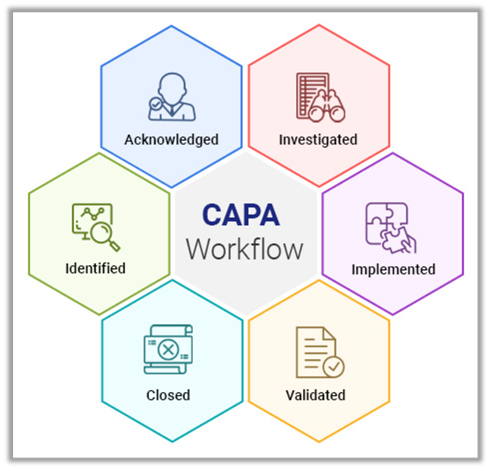
The highlight of digital CAPA management can be best explained by the definition provided by FDA:
“The purpose of the corrective and preventive action subsystem is to collect information, analyze information, identify and investigate product and quality problems, and take appropriate and effective corrective and/or preventive action to prevent their recurrence. Verifying or validating corrective and preventive actions, communicating corrective and preventive action activities to responsible people, providing relevant information for management review, and documenting these activities are essential in dealing effectively with product and quality problems, preventing their recurrence, and preventing or minimizing device failures. One of the most important quality system elements is the corrective and preventive action subsystem.”
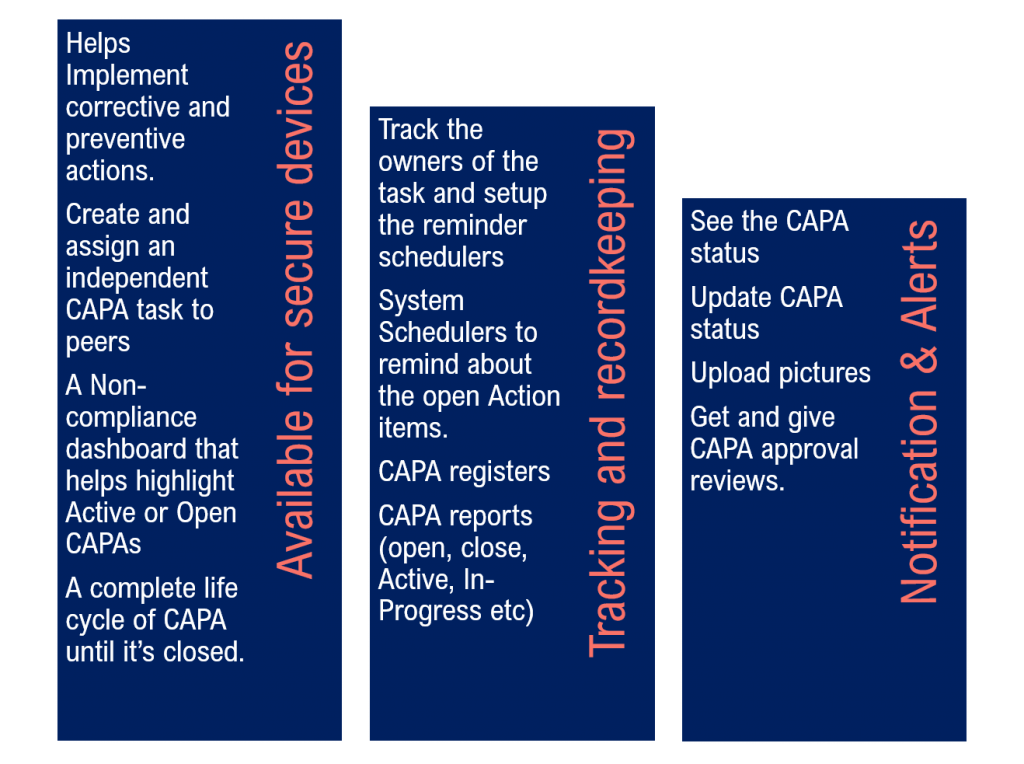
3 ways to that simplify the CAPA Process with software management
- A corrective action is a more serious nonconformity that carries risk which may prove to be an incident. This can be due to an adverse event or may because the number of departments it affects. These must be recorded, along with the steps that are taken to prevent future occurrences.
- A preventative action is one in which no problem currently exists, but there is a potential risk for an adverse event. The idea is to consider “what-if” possibilities and prevent them from happening.
- A developmental action process is one in which preventative action is taken to avoid adverse events in new products, processes, or procedures.
CAPA Pros and cons
Pros:
- Provides a structured platform which will allow systemic investigation of adverse events
- Offers a means by which to develop a permanent corrective action, thereby reducing the propensity for adverse event recurrence
- Provides documentation that corrective actions are effective
Cons:
- Can be tedious and exhausting to manage
- Cost of quality may be difficult to track or monitor when disorganization is present
- Poor management can lead to inconsistent data, confusion, non-compliance, and hesitancy in taking action when needed
- Misunderstandings about the CAPA process can lead to clogged processes that make it difficult to report across some or all departments
Is your organization searching for a better way to manage your CAPA process, then see our software module EHS management of CAPA process.



How interesting that you talk about this structured platform for the investigation of adverse events. I am starting a new consulting business this year. I will contact with you about software.